| |
 |
Should you encounter any unexpected behaviour,
please let us know.
|
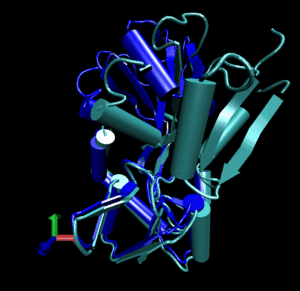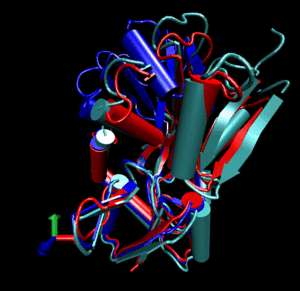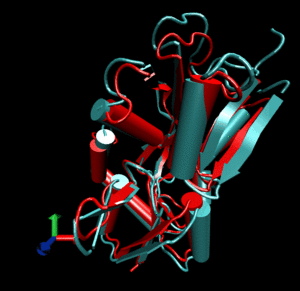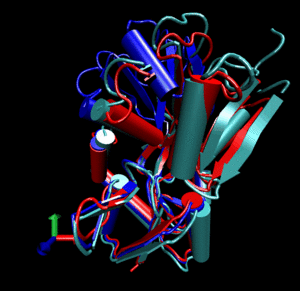
Glutamine binding protein
|
Upon ligand binding, Glutamine binding protein undergoes a significant conformational change between its
open (blue cartoon, 1ggg).
and its closed form (red cartoon, 1wdn).
Application of a combined normal mode perturbation of dq=200 in the direction of mode 7 and of dq=40
in the direction of mode 8 brings the open form near the closed form (silver cartoon).
Using this perturbed template in molecular replacement (AMoRe) it becomes
possible to solve the structure of the closed form. The final R-free after CNS refinement of
the perturbed model is 47.1, compared to 54.0 when using the open form as a template.
Note that in this case a simulated annealing step had to be applied during refinement.
(see also Molmovdb
for an analysis of this protein's movement).
|
click to enlarge (3.2MB)
If you find results from this site helpful for your research, please cite one of our papers:
elNémo
is maintained by Yves-Henri Sanejouand.
It was developed
by Karsten Suhre.
Between 2003 and 2014, it was hosted by IGS (Marseille).
Last modification: April 8th, 2025.
|
|
 |
 |
|