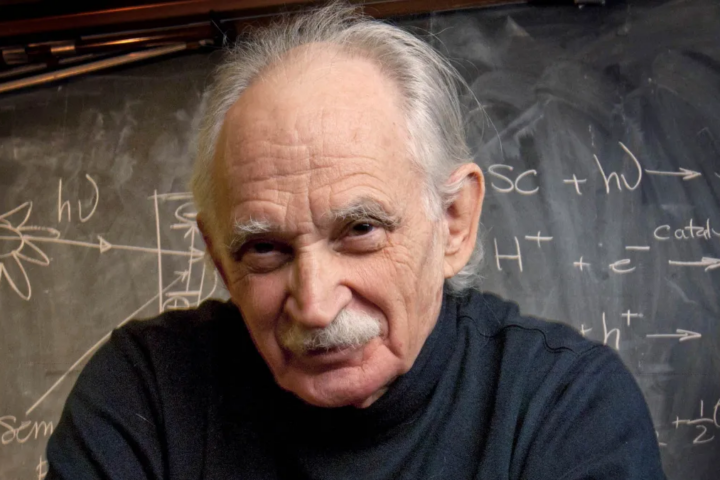CEISAM Institute
Research institute in Molecular Chemistry
UMR CNRS 6230
The CEISAM Institute (Interdisiplnary Chemistry: Synthesis, Analysis, Modelling) was founded in 2008, in order to structure Nantes Molecular Chemistry (theoretical chemistry, organic / organometallic synthesis, analytical chemistry & physicochemistry).
Latest News
DCO SCF Jean-Pierre Sauvage prize awarded to Simon Pascal
The Division of Organic Chemistry of the French Chemical Society has awarded the “Jean-Pierre Sauvage” Young Researcher prize to Simon Pascal. His research stands out in the field of synthesis of “exotic” organic dyes and the modulation of their optical properties, particularly in the near-infrared range, opening up new perspectives in areas such as bio-imaging...
CNRS bronze medal : Morgane Vacher
As a reminder, Morgane VACHER is pursuing a very promising career in the ModES team following her recruitment in 2019. She was awarded an ERC starting grant in 2022 and has co-authored more than 48 international publications and 6 book chapters. Morgane has also been invited to speak at some twenty leading international conferences. This...
Tribute : Allen Joseph Bard, the pioneer and father of electrochemistry
Allen Joseph Bard, the pioneer and father of electrochemistry, passed away on February 11, 2024 in Austin, Texas. He was 90 years old and had a 63-year career at The University of Texas at Austin. For most of his students, postdoc and co-workers, he was the greatest electrochemist of several generations. He will be highly...
Next events
Our research teams
This Institute is organized into five research teams covering coherent areas of activity but which are based on different and highly complementary segments, giving rise to original transversal projects.
On the basis of fundamental and specific expertise, CEISAM is positioned in a certain number of niches of excellence at the interface with the fields of Health, Food chemistry and Materials, through privileged interactions at regional, national and international levels.
Platforms and equipment
To support our research activities, the CEISAM Institute owns many specialist equipments.
These instruments and equipment chains, sometimes intégrated into platforms, aim to synthesize, model, prepare and characterize organic or hybrid items. The European Union, the CNRS and the "Pays de la Loire" region have largely contributed to co-financing these platforms and equipments in particular in NMR, Mass Spectrometry, Molecular Modeling, Optical spectroscopies and microscopy.